The increasing use of renewable energies means that more and more electricity being generated – whether by large wind and solar farms or by small and medium-sized privately owned PV installations – has to be temporarily stored decentralized. Since the lithium-ion batteries frequently used for this purpose suffer from a number of disadvantages – among other things with respect to the storage of energy over longer periods of time or decreasing storage capacities at low temperatures or over the course of use – alternative technologies are being sought. What are known as “redox flow batteries” or wet-cell batteries are already in use.
Redox flow batteries are elaborately constructed liquid batteries in which electrolytes, often based on vanadium, are circulated by means of pumps. The technology is seen as having great potential as a storage system for renewable energy from solar photovoltaic and wind farms or rooftop systems. The energy conversion takes place in an electrochemical cell which is divided into two half cells. Figure 1 shows the general operating principle of redox flow batteries.
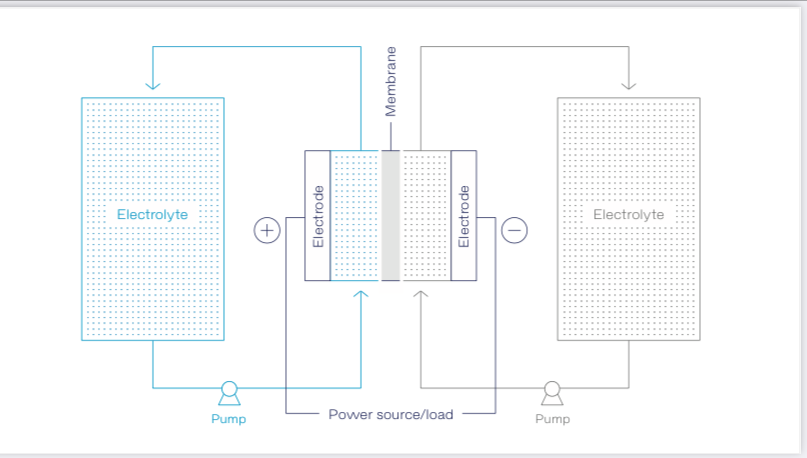
The half cells are separated from each other by an ion-permeable membrane or separator, so that the liquids in the half cells mix as little as possible. Like in fuel cells, the individual stacks can be combined in series to create a “cell stack” that typically comprises flow frames, bipolar plates, electrode felts and gaskets (fig. 2).
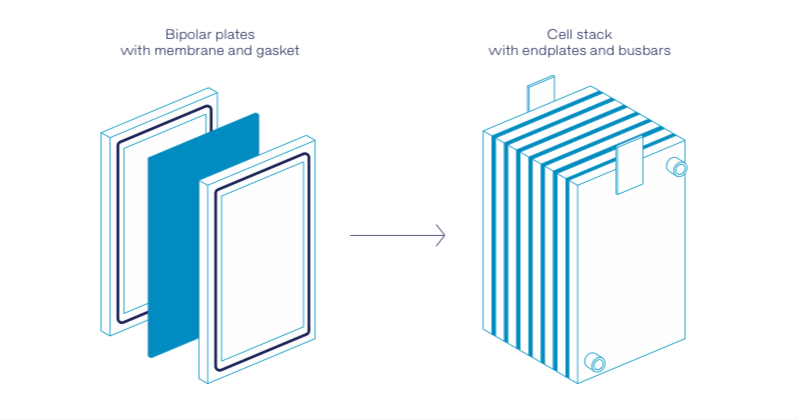
So far, designing these battery stacks has been a real challenge as the aggressive electrolytes can stress the gaskets and sealants used. If the sealing material is not resistant enough, it can either swell or degrade and lead to undesired leakages from the stacks and the entire battery assembly. Potting resins, adhesives and sealants from Wevo can withstand these conditions – as demonstrated by a series of tests conducted by the company in cooperation with the Fraunhofer Institute for Chemical Technology (ICT) in Pfinztal, Germany. (Read more in FAPU issue 118, March 2021 page 33-36












